For most parents, their child’s fifth birthday marks a happy milestone. But for Tommy Pham, it is a bittersweet one for his son Raiden, who was born with a mutation in the UBA5 gene, which is critical for protein manufacture and processing within cells. As a result, Raiden cannot sit, walk, talk, or feed himself, and requires 24/7 care.
“He’s just lying there like he’s a baby, and he just turned five. It’s a heartbreak. He’s supposed to be running, and playing with kids, or in school, but he can’t do any of that.”
Tommy Pham
When Raiden’s UBA5 mutation was diagnosed in 2021, only about 30 cases were being studied worldwide, a number that has only increased to 38 as of 2024. Since then, a handful of other impacted children have been identified in social media groups, with their conditions being debilitating and life-threatening: most have severe developmental delay, microcephaly, growth failure, and epilepsy [1].
Raiden’s uncertain future, combined with a lack of science surrounding this ultra-rare disease, spurred Tommy and his wife, Linda, to form the Raiden Science Foundation (RSF). The foundation networks with scientists and fundraises to support research on UBA5 Disorder, including building a patient network, creating animal models, and repurposing drugs, and creating a gene therapy.
Beyond Animal Models: Creating a Human “Brain in a Dish”
RSF, through a grant in partnership with CURE Epilepsy, is also banking on another method to directly study the effects of UBA5 mutations in human brain cells: brain organoids. Grown in the laboratory from skin cells, brain organoids are three-dimensional structures designed to mimic the cellular composition and architecture of the developing brain, allowing researchers to study the brain “in a dish” in the lab. They contain many types of brain cells, like neurons and astrocytes, which connect with each other as cells do in the human brain. Growing organoids involves a multi-step process of transforming skin cells into induced pluripotent stem cells (iPSCs), which have the potential to be made into any kind of cell in the body. Scientists can then direct the iPSCs to grow into different sorts of brain cells, that can be maintained and studied for over 100 days in laboratory dishes [2].
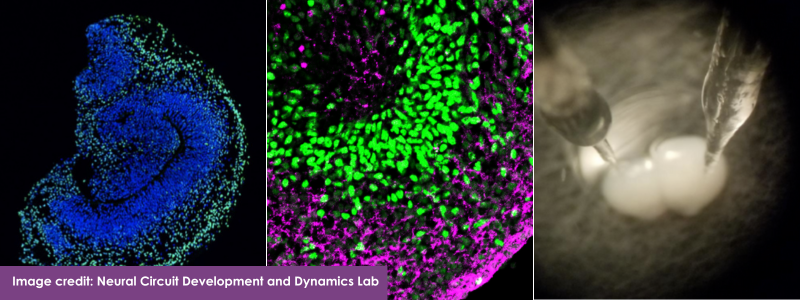
While brain organoids do not show the elaborate structures found in a human brain, they can be produced cost-effectively in large enough quantities to support different types of experiments, including those exploring potential drug or gene therapies. They also offer personalized insights, since they contain a patient’s entire genetic code, including their particular disease-related mutation. Because not all mutations of the same gene act alike, brain organoids could help pave the way toward personalized medicines.
While brain organoids can examine a mutation’s effects inside human cells, providing great insight to the drug discovery process, they cannot replicate the complicated consequences seen in a boy like Raiden. Thus, brain organoids complement, but do not replace, other experimental approaches such as development of animal models of genetic epilepsies.
“There’s so much more to learn, but because the population is so small, and because it’s hard to get access to brain tissue, there needs to be a way to understand brain development for those affected by UBA5. It’s an important part of disease modeling.”
Tommy Pham
It’s Personal: Brain Organoids and Personalized Medicine
CURE Epilepsy also funds brain organoid research related to early-onset epilepsy and intellectual disability due to SCN8A gene mutations. The SCN8A gene encodes a sodium channel involved in transmitting electrical activity in the brain. Dr. Ranmal Samarasinghe of the University of California, Los Angeles (UCLA) has been awarded a grant to study a specific SCN8A mutation with brain organoids, in a brain-region specific way.
Samarasinghe and his team used iPSCs from patients with SCN8A mutations to grow cortical or hippocampal brain organoids to study how this mutation affects their function. Preliminary results show that SCN8A mutations affect these brain regions very differently; cortical organoids showed hyperexcitability similar to seizure activity while hippocampal organoids did not. The abnormal activity patterns and disrupted excitatory cell numbers in the hippocampal organoids suggest this region contributes to intellectual disability independent of seizure activity.
CURE Epilepsy has also funded Dr. Colin McCrimmon, a trainee of Samarsinghe’s, with an early-career Taking Flight Award. He will continue to study the brain region-specific effects of SCN8A mutations and explore whether certain anti-seizure medications can remedy any of the deficits found in these organoids.
Ultimately, lessons learned from brain organoid modelling of UBA5 or SCN8A disorders will inform and expedite research for many other rare epilepsy conditions. Scientists will use their hard-won expertise to catalyze research for other disorders, and more efficiently catalog the cellular consequences of a range of mutations – bringing us closer to personalized medicine.
“We’re helping to develop a platform and skills for other rare diseases, too. We want to fight for expanding knowledge, for research, for potential care in the future. The best way to describe it is that we’re fighting for hope.

Literature Cited:
- Briere LC, Walker MA, High FA, Cooper C, Rogers CA, Callahan CJ, Ishimura R, Ichimura Y, Caruso PA, Sharma N, Brokamp E, Koziura ME, Mohammad SS, Dale RC, Riley LG; Undiagnosed Diseases Network; Phillips JA, Komatsu M, Sweetser DA. A description of novel variants and review of phenotypic spectrum in UBA5-related early epileptic encephalopathy. Cold Spring Harb Mol Case Stud. 2021 Jun 11;7(3):a005827. doi: 10.1101/mcs.a005827. PMID: 33811063; PMCID: PMC8208045.
- The Use of Organoids in Epilepsy Research [Webinar]. CURE Epilepsy. https://www.youtube.com/watch?v=BX1mg70T8L4.